The underestimated mutation potential of retrogenes
A new study resulting from a collaboration between the Max Planck Institute for Evolutionary Biology in Plön and the Chinese Academy of Sciences in Beijing shows that the potential genetic burden of mutations arising from retrogenes is significantly greater than originally thought.
Update September 2021: The publication mentioned is now occasionally cited in certain forums to classify vaccination against the COVID-19 virus as fundamentally dangerous. We would like to comment on this as follows:
It is important to know that the rewriting of mRNA into the genome is a natural process and presumably a large part or all of the mRNA can be rewritten into the genome. This process occurs continuously. Viral mRNA can also potentially be integrated, meaning that even if one is infected with coronavirus or another virus, there is a possibility that this will have an impact on mutations in the genome. In the mass of these natural processes, the potential additional effect of mRNA vaccination hardly matters. Also, only a small number of cells will ever be affected, and if they carry a mutation, they will be quickly replaced by other cells. It is not to be expected that injected mRNA will be taken over into the germ cells, i.e. a possible inheritance can be practically excluded.
Thus, taking this background into account, it can be said with a very high degree of certainty that the potential danger posed by RNA written back (retrogenes) in connection with vaccination is significantly lower than the danger that can be posed by infection with the coronavirus, or that posed by natural cell processes.
Genetic information is stored in DNA and transcribed as mRNA. The mRNA is usually translated into proteins. However, it has long been known that mRNA can also be reverse transcribed to DNA and integrated back into the genome. Such cases are referred to as retrogenes. In an article published in Proceedings of the National Academy of Sciences USA (PNAS), a team from the Max Planck Institute for Evolutionary Biology in Plön and the Zoological Institute of the Chinese Academy of Sciences in Beijing now reports that this process was previously underestimated by at least a factor of one thousand and that it is an important new mutation mechanism.
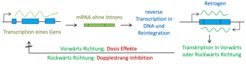
Retrogenes are detected by an optimized algorithm
There are two main reasons for this. On one hand, the common search algorithms used in genome sequence analysis do not usually take new insertions of retrogenes into account. These therefore remain hidden in the mass of data. Only with an optimized algorithm (like the one developed by the authors) can these insertions be systematically discovered. On the other hand, the authors showed that most of the insertions are relatively short-lived. In previous genome comparisons between species, they appear to be comparatively rare.
Mutation by retrogenes is usually harmful
For this most recent study, it was therefore crucial to examine populations that have only recently developed. The authors found that mouse populations that have been separated for only about 3000 years carry different retrogenes (i.e. in each population, retrogenes emerge at a very high rate but are also lost again comparatively quickly). This is because retrogenes can be harmful – even if they are integrated into non-coding DNA. If retrogenes are transcribed back into mRNA (as is the case for most of them), this new mRNA can negatively influence the mRNA of the gene from which they originated. The retrogene thus acts as a regulatory mutation, which is usually harmful. The authors show that the genetic burden of this mechanism is higher than that of the point mutations, which until now have been the primary focus of investigations. They therefore suggest that the search for disease-causing mutations also take the retrogene mechanism into account.
